Introduction
In the biopharmaceutical industry, many biological drugs are produced in mammalian cells. Cell line development (CLD) is used to determine which cell lines have the highest recombinant protein production and are also stable during large-scale manufacturing.
In the CLD workflow, cell lines undergo incremental scaling of culture from static to shaker flask expansion and further scaling up in bioreactors. Cell culture in early CLD is currently limited to static format as the size of the well plates and number of cells limit the ability to agitate the culture.
Introduction
In the biopharmaceutical industry, many biological drugs are produced in mammalian cells. Cell line development (CLD) is used to determine which cell lines have the highest recombinant protein production and are also stable during large-scale manufacturing.
In the CLD workflow, cell lines undergo incremental scaling of culture from static to shaker flask expansion and further scaling up in bioreactors. Cell culture in early CLD is currently limited to static format as the size of the well plates and number of cells limit the ability to agitate the culture.

Dynamic High-throuput screening in early stage
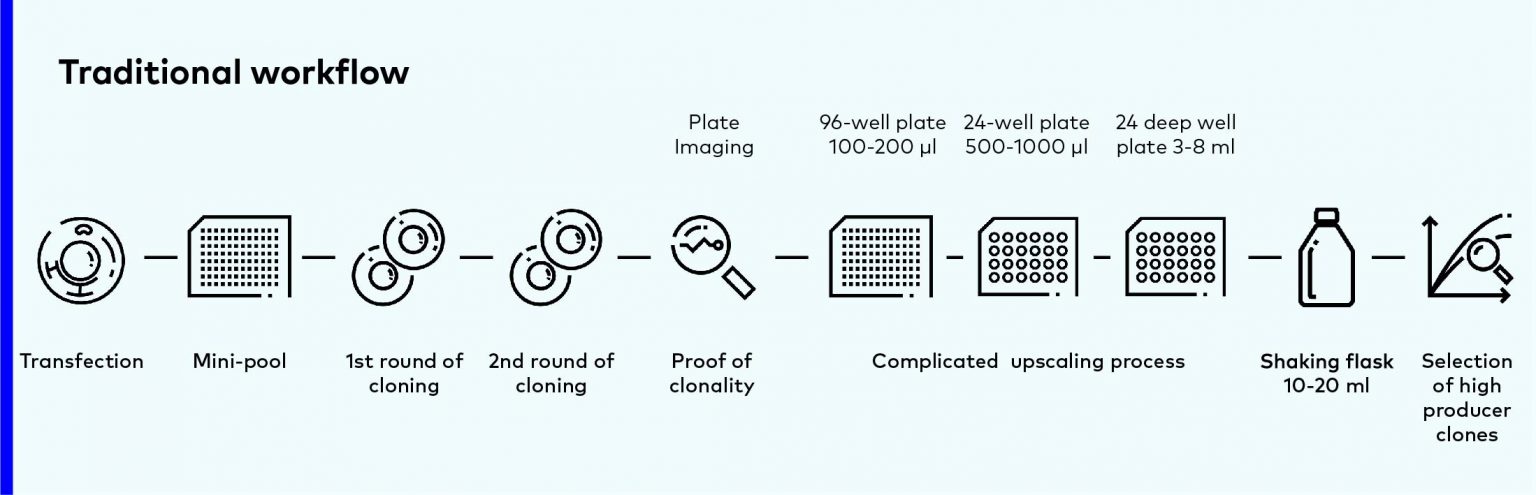
Traditional workflow
Traditionally, CLD starts from single cell isolation in post-transfection pools. After clones selection, the outstanding cells are transferred to larger and larger scale of cultivation system, and it almost takes several weeks.
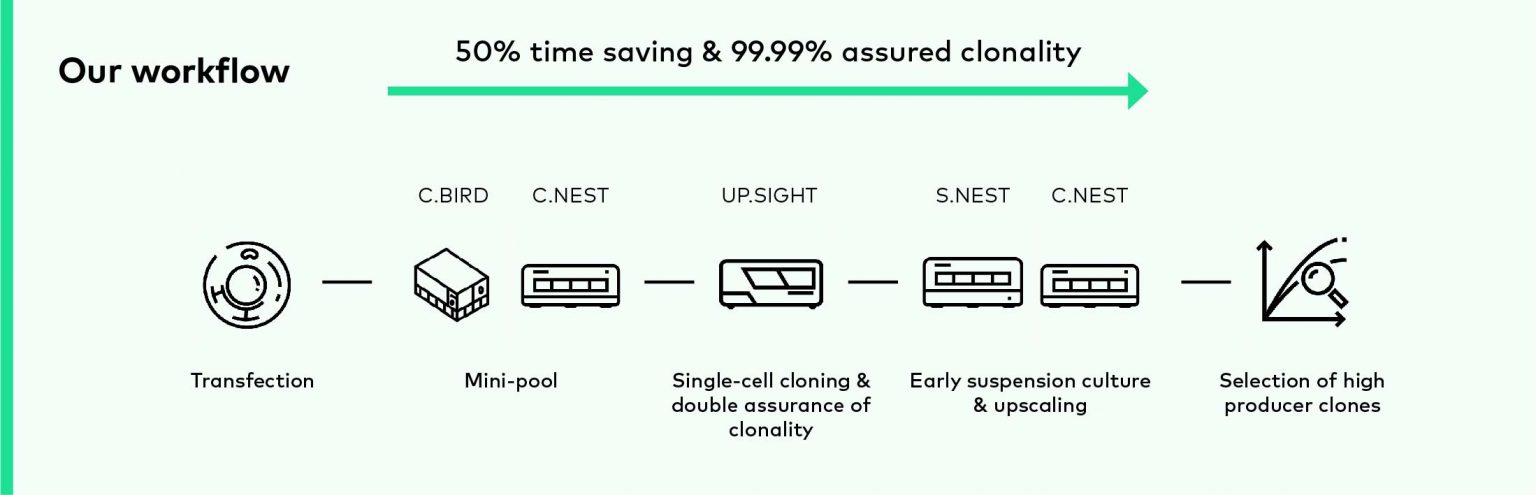
CYTENA workflow
To improve and accelerate the cell line development, we offer the comprehensive product portfolio for significantly increasing the speed, quality, and efficiency of your cell line development workflows.
01.
Comparability with
Shaker-flask

CHO-KI
CHO-S
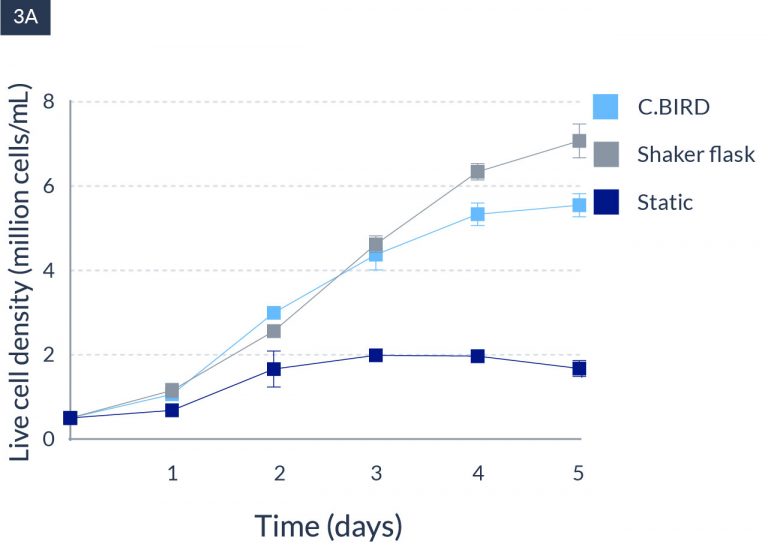
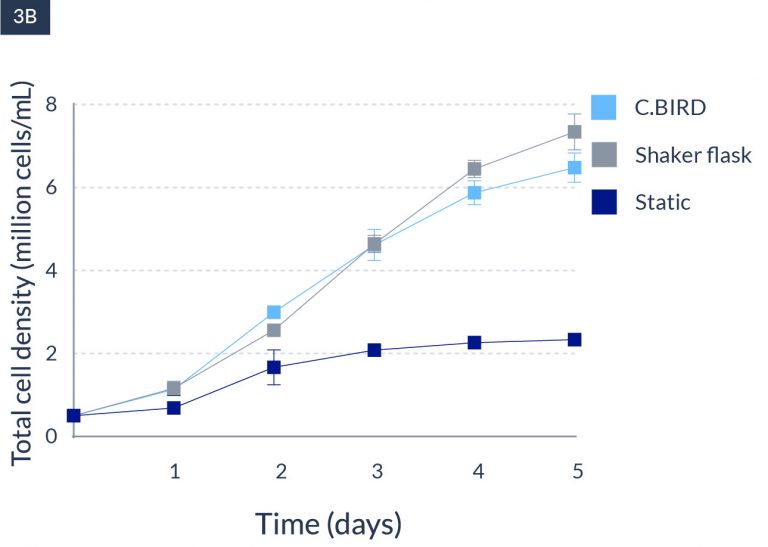
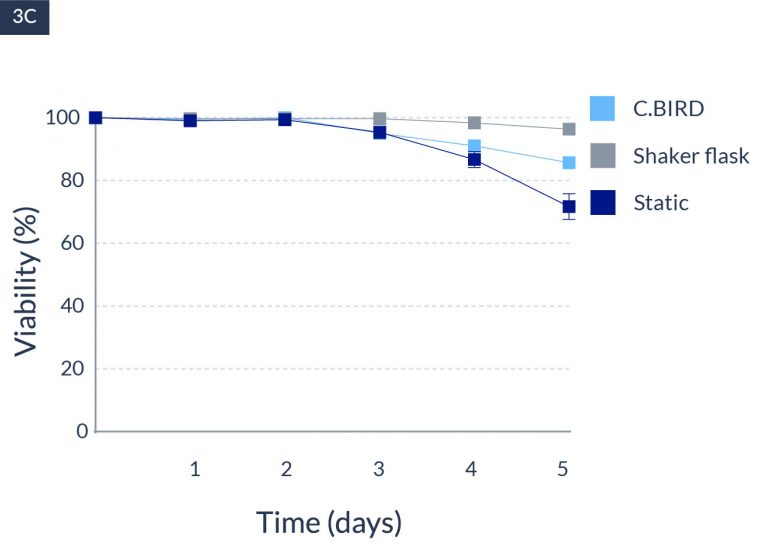
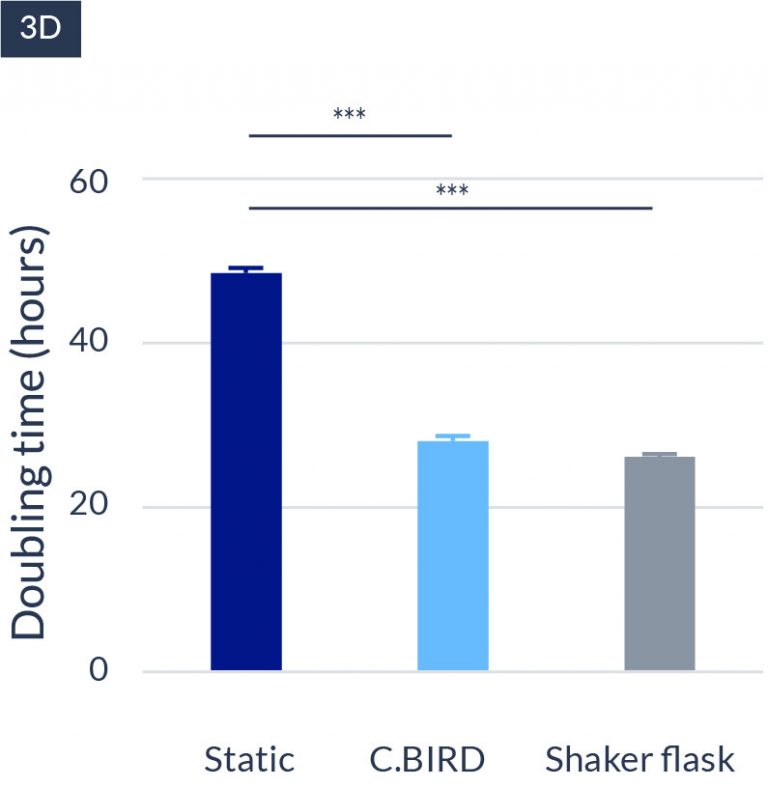
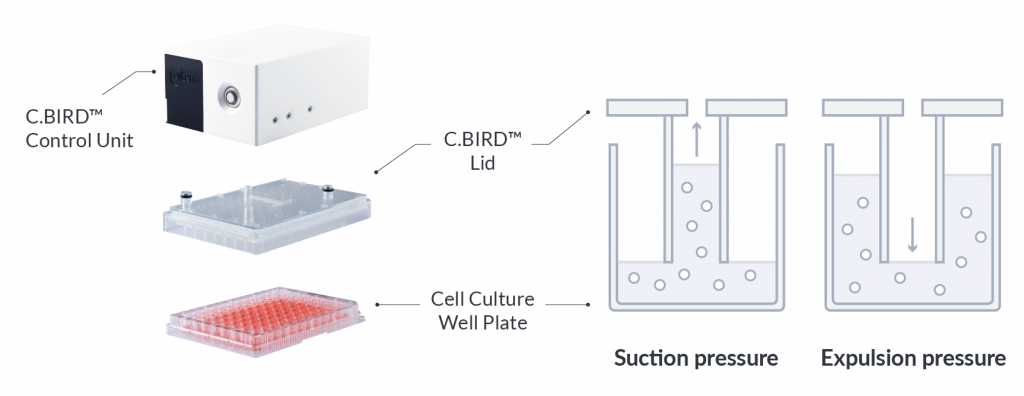
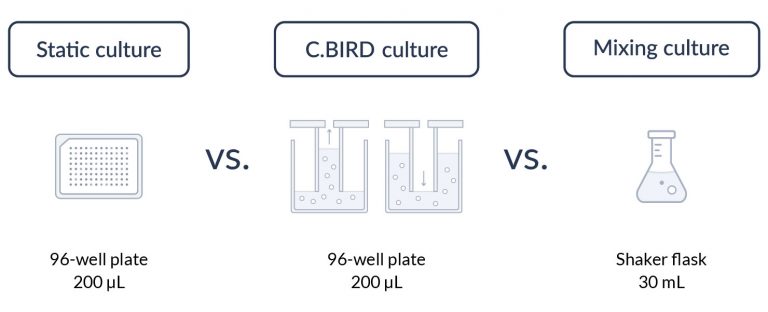
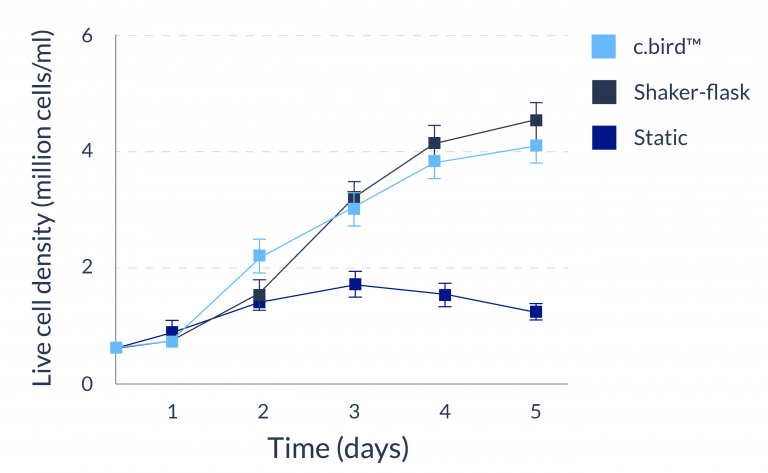
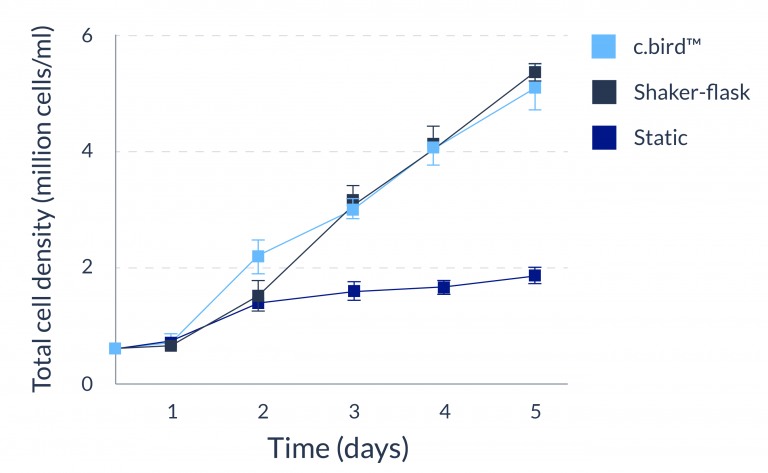
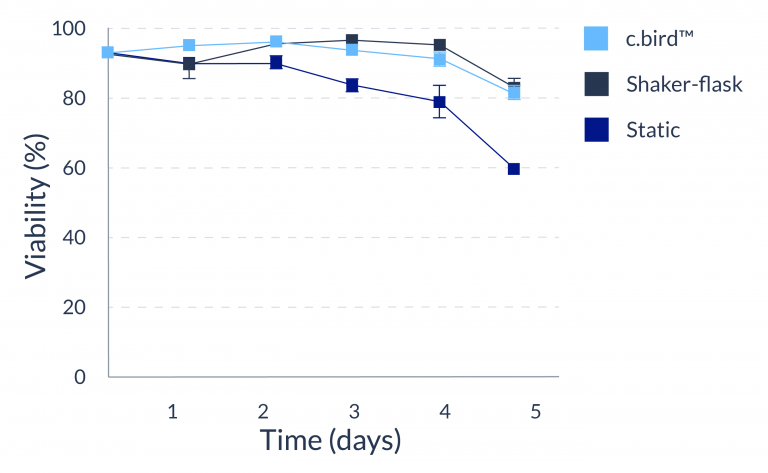
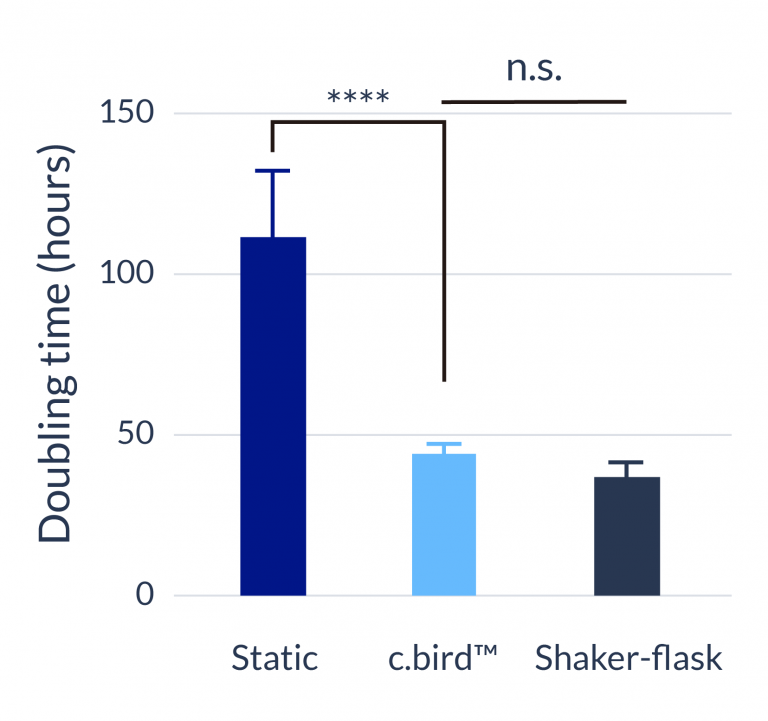
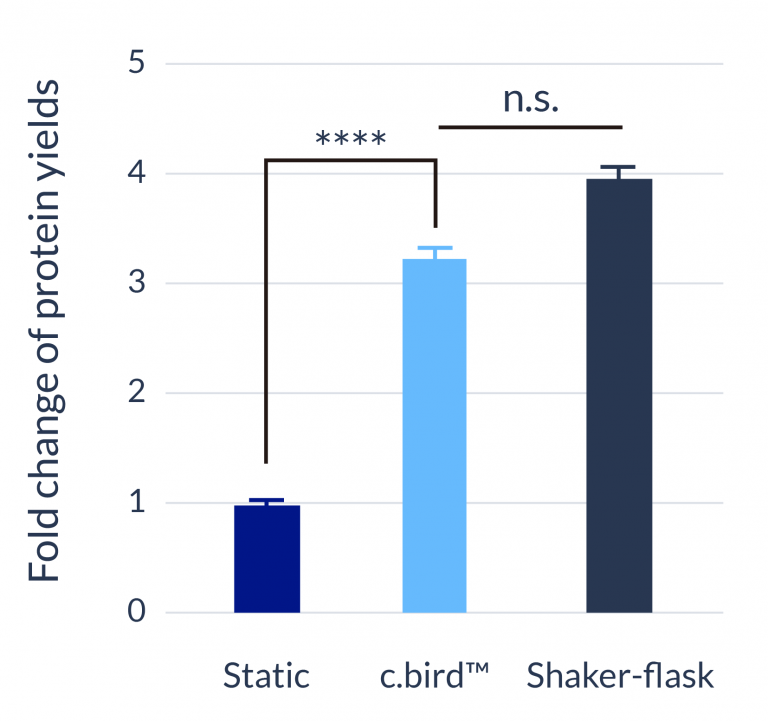
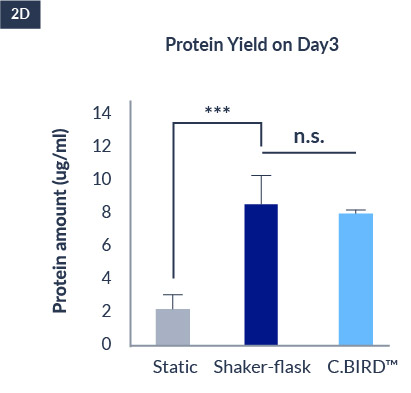
C.BIRD enables early transition to suspension cell culture with higher cell growth rate in standard 96-well plates, potentially providing better translation to large-scale shaker-flask/bioreactor conditions for later CLD processes.
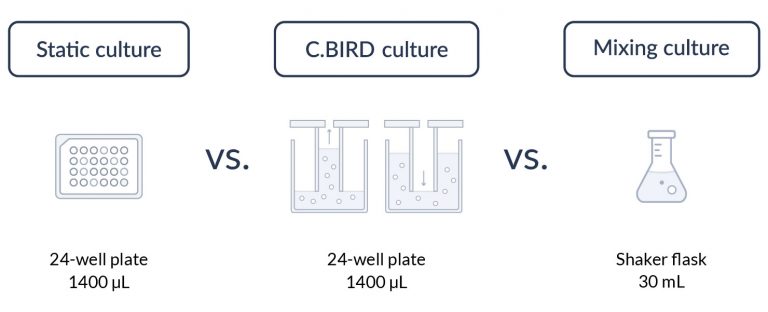
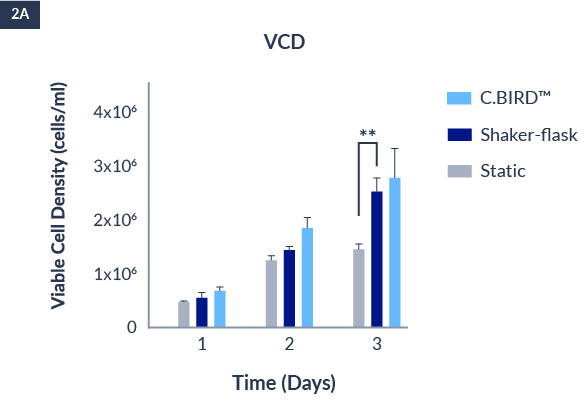
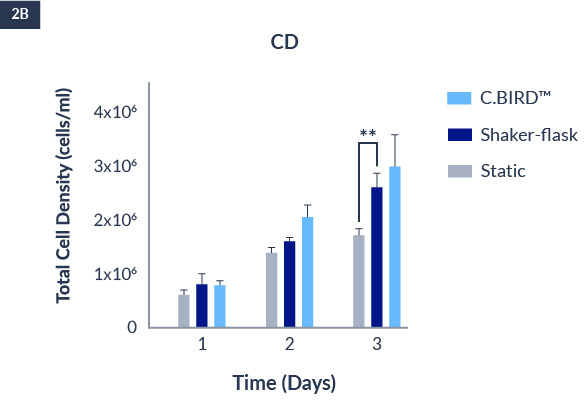
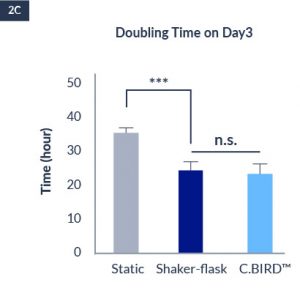
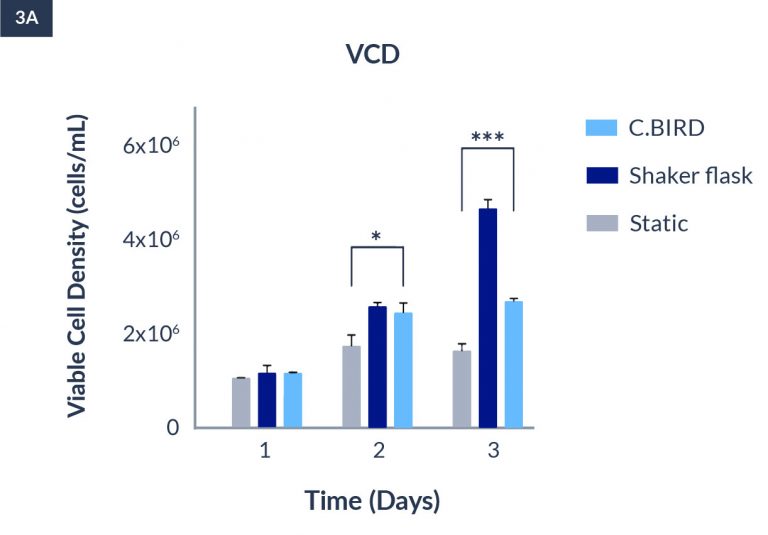
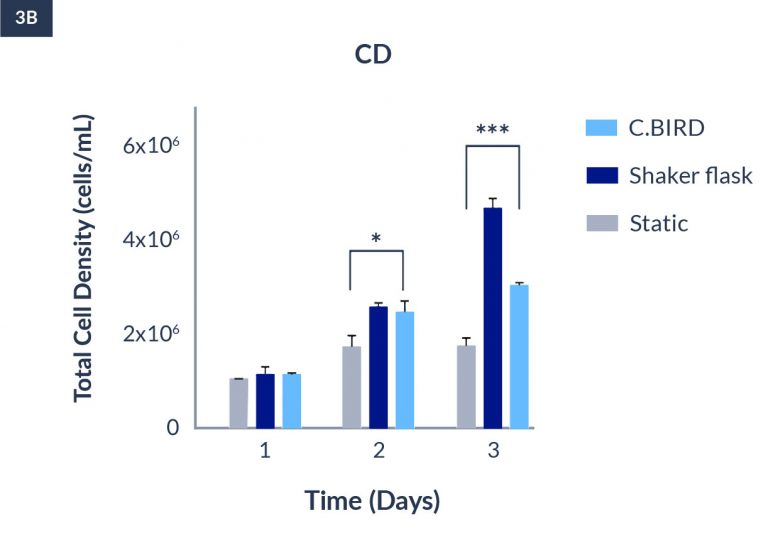
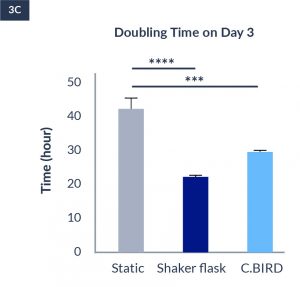
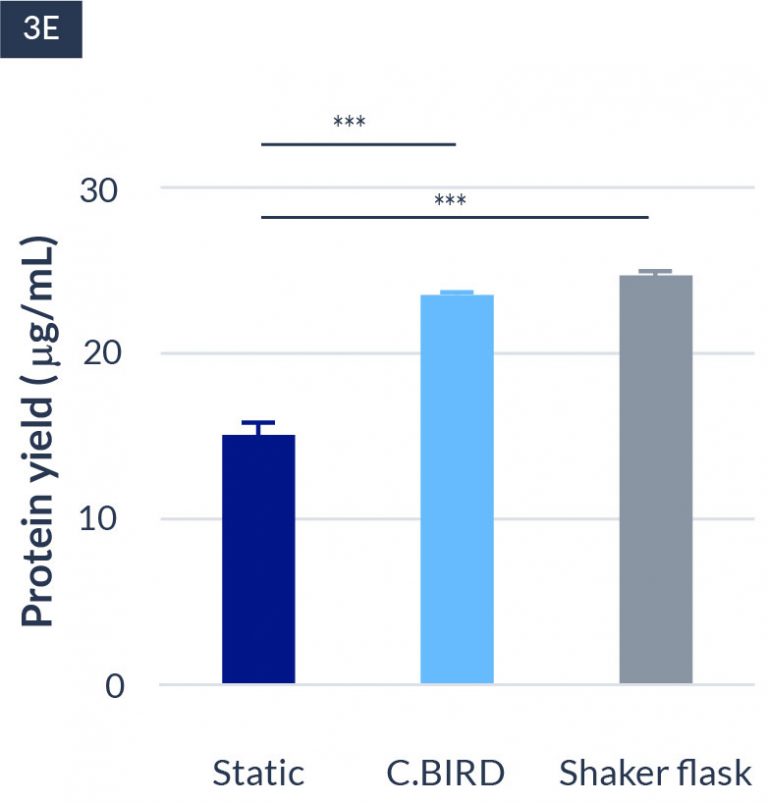
C.BIRD method on 24-well plates finalizes the workflow of CLD and offers a more amenable culture environment for suspension cell lines.
02.
High-yield protein production
The high consistency of cell growth and protein yield profiles enables better predictability of biological profiles of the clones at early CLD stages. It also avoids additional time and costs spent on picking undesirable clones.
CHO-KI
CHO-S
96-well format
24-well format
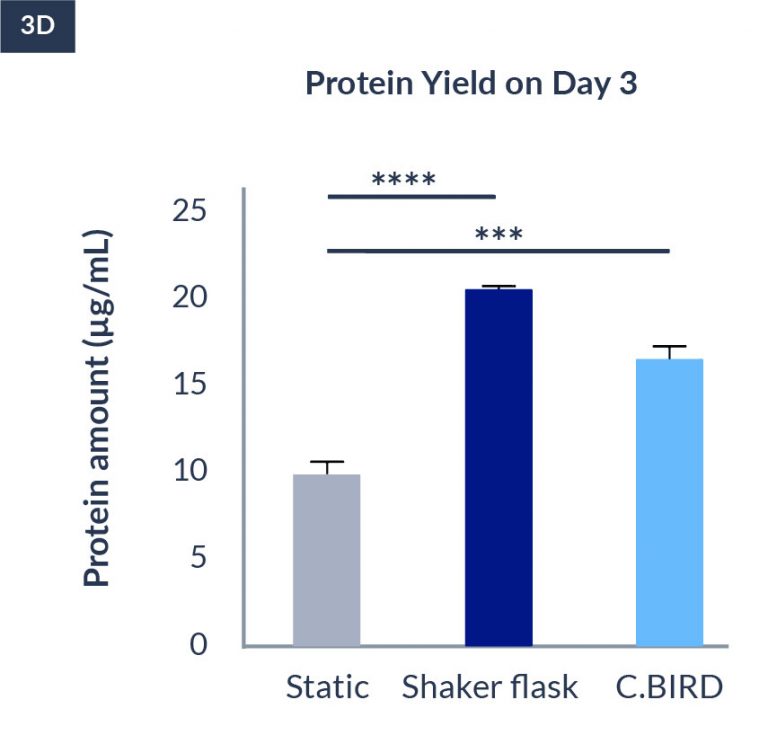
CHO-KI
96-well format

CHO-S
96-well format

CHO-KI
24-well format

CHO-S
96-well format






