Introduction
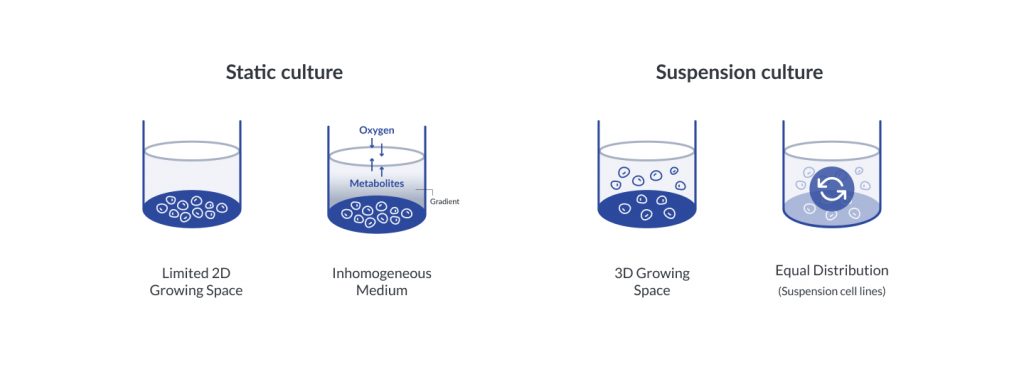
Traditional cell culture uses the monolayer culture which is also a standard procedure. However, the 2D cell culture method is not similar to the in-vivo microenvironments. 3D cell culture mimics the in-vivo microenvironments, which can improve cell growing with in vitro model.
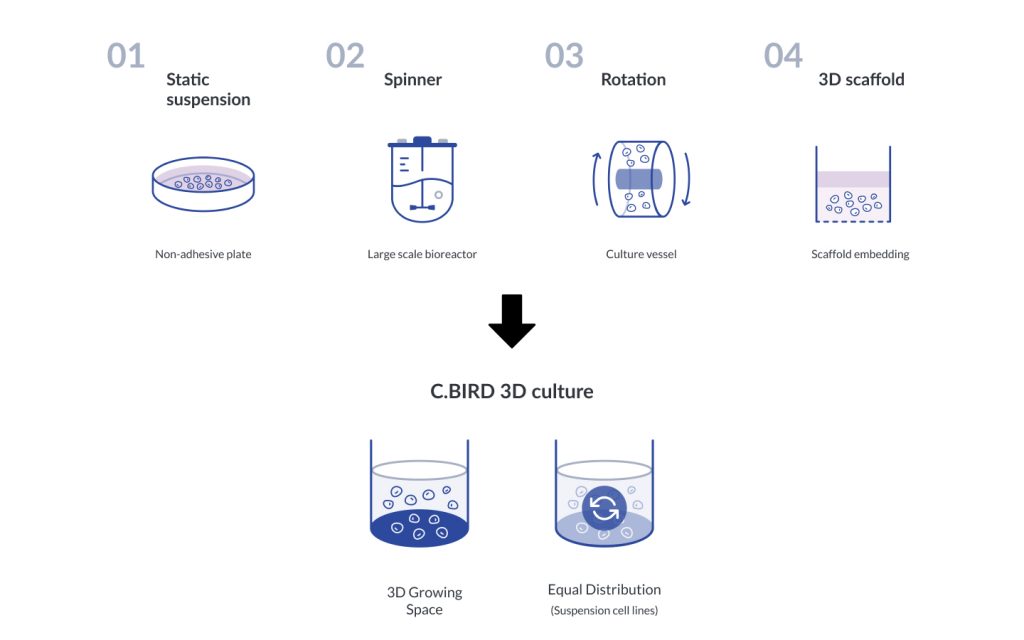
To achieve the purpose of 3D spheroid cell culture, numerous methods have been used to establish the culture environments such as suspension culture of non-adhesive plate, spinner bioreactor, rotating bioreactor, 3D scaffold etc.
C.BIRD offers continuous mixing as a multiple pipetting system, which can keep cells in a low adhesion status. Cells will grow and aggregate into a spheroid in the standard 96/24-well plates.
Introduction
Traditional cell culture uses the monolayer culture which is also a standard procedure. However, the 2D cell culture method is not similar to the in-vivo microenvironments. 3D cell culture mimics the in-vivo microenvironments, which can improve cell growing with in vitro model.
To achieve the purpose of 3D spheroid cell culture, numerous methods have been used to establish the culture environments such as suspension culture of non-adhesive plate, spinner bioreactor, rotating bioreactor, 3D scaffold etc.
C.BIRD offers continuous mixing as a multiple pipetting system, which can keep cells in a low adhesion status. Cells will grow and aggregate into a spheroid in the standard 96/24-well plates.

Traditional methods of 3D culture
Our solution
Performance data
An Innovative 3D Cell Culture System for Tumor Spheroid
Spheroid culture, an in vitro 3D cell culture system, provides cell-cell and cell-extracellular matrix (ECM) interaction networks to retain cellular phenotype through signaling. It breaks restrictions in recapitulating in vivo microenvironments and is emerging as a powerful tool for therapeutic development, including drug screening and cancer research. In recent studies, gene and physiological expressions in 3D cell spheroids are much closer to clinical expression profiles than those seen in 2D cell monolayers.

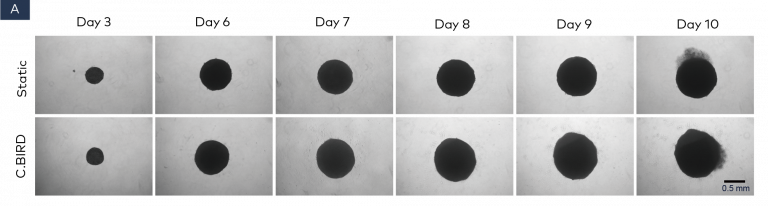
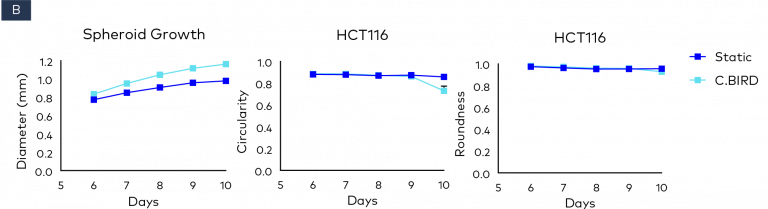
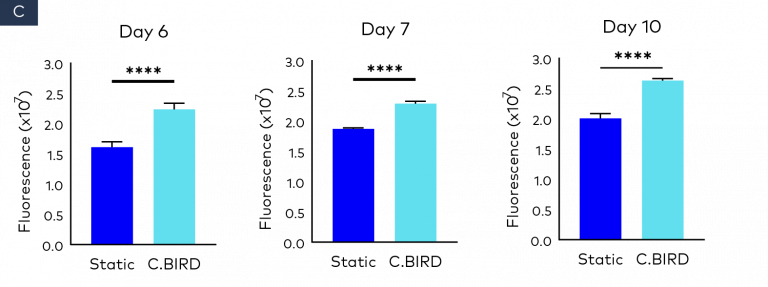
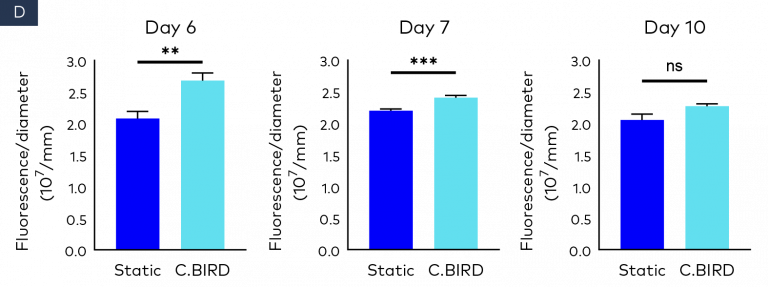
Figure 2. Comparison of spheroid growth and cell health of colon cancer HCT116 spheroids in 96-well, ultra-low attachment plates in static and C.BIRD culture. (A) Spheroid morphology of HCT116 tumor cell lines formed from 3 to 10 days was observed under microscopy. Scale bar = 0.5 mm. (B) Growth kinetics, circularity and roundness of spheroids were evaluated over a period of 10 days. The analysis was performed using ImageJ software, and data represent mean ± SEM of six replicates for each group. (C and D) Spheroid cell health was assessed using PrestoBlue Cell Viability Reagent. After 6, 7 and 10 days of spheroid culture, 20 μL of PrestoBlue Cell Viability Reagent was added to each well, which were then incubated at 37°C and 5% CO2 for an additional 3 hours before being read on a fluorescence-based microplate reader (Ex/Em ~560/590 nm). Cell viability was measured as fluorescence signals in each group with six replicates. Fluorescence signals were normalized by spheroid diameter; a higher ratio (fluorescence/diameter) indicates healthier spheroids. The significance of P values is listed as: P ≤ 0.01 (**), P ≤ 0.001 (***) and P ≤ 0.0001 (****). Data are shown as mean ± SEM.