Drug Screening
Real-time DO, pH, OCR, ECAR monitoring for in vitro drug screening
Introduction
Preclinical drug screening is a crucial step in the development of new pharmaceutical compounds or therapeutic agents for treating, preventing, or managing various medical conditions. The development process generally begins with identifying drug candidates that may influence the disease pathway of interest. Once drug candidates are identified, drug screening is performed to validate the potential of these candidates by evaluating their potential efficacy, cytotoxicity, and possible side effects.
Challenges in
Traditional
Workflows
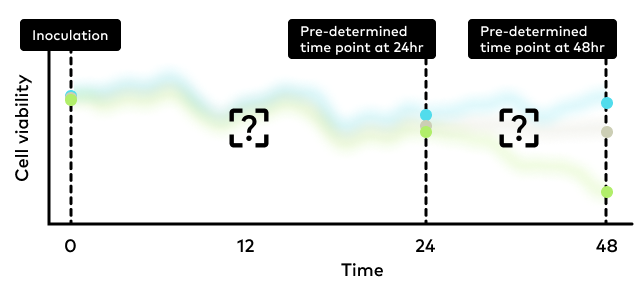
The process of drug screening is inherently complex. One major challenge is the design of experiments involving endpoint assays. Ideally, these experiments should provide reliable and useful information about how the drug candidates modulate the underlaying disease pathway. However, the life cycle of any chosen cell line could influence assay results, on top of any effects from drug treatment, making it difficult to design experiments free of bias.
Furthermore, cytotoxicity and efficacy of the drugs are often assessed by cell viability assay at pre-determined timepoints after treating the cultivated cells. This assay involves sacrificing samples for measurement and dyeing cells to quantify viability. The manual manipulation here is not only labor intensive and time consuming, but also increases the risk of contamination for the entire experiment.
A New Solution-
S.NEST
Microbioreactor
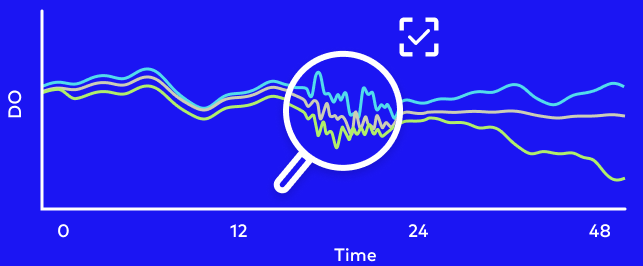
S.NEST microbioreactors revolutionize the drug screening process by replacing endpoint assays with real-time monitoring of key indicators in incubation chambers. These chambers provide optimal culture environments with unique chamber design and in-well reciprocal mixing. During the culturing process, researchers can measure pH, dissolved oxygen level (DO), oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) in every well, as frequently as every 10 minutes.
Unlike the traditional screening process, S.NEST mitigates the need for any manual operation for measurements and can easily detect any time-dependent changes. Integrating S.NEST into a new workflow can therefore accelerate screening processes, while reducing labor time, operational costs, and risk of contamination.
Performance Data
Case in Point -
Experimental
Design Involving
End-Point Cell
Viability
When researchers employ conventional end-point assays, they may have to design experiments iteratively with varying measurement timepoints, until the most informative timepoints are found.
An example experiment is described here to illustrate this pitfall: A549 cells were seeded at a density of 5 × 104 cells/well and treated with Methotrexate (MTX) and Staurosporine (SSP), the therapeutic drugs. A Prestoblue cell viability assay was performed to assess the cytotoxicity and efficacy of the drugs at the pre-chosen timepoints of 24 and 48 hours of treatment. The Prestoblue cell viability assay showed that the cell viability was significantly reduced at 24 and 48 h after treatment by 100 nM SSP, but not by 100 nM MTX.
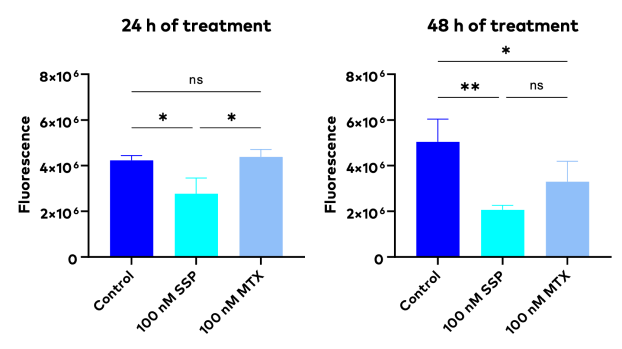
The difference in cell viability between different treatments was significant at 24h but not at 48h after treatment. (The P values are indicated as: P ≤ 0.05 (*), P ≤ 0.01 (**). ns indicates no significant difference. Data were shown as mean ± SD)
From the results of this experiment, researchers can only narrow down but not pinpoint the treatment reaction times, because the actual reaction times fall outside of the range of the tested timepoints. To pinpoint the reaction times for subsequent testing, researchers would have to repeat this experiment iteratively with different timepoints. This protracted, labor-intensive, and costly endeavor underscores the time-consuming nature of drug screening processes.
New Workflow
with Real-Time
DO/pH and
OCR/ECAR
Incorporating S.NEST microbioreactors is very easy and will result in a new screening workflow that is very straightforward. Just like in the conventional workflow, cell culture can be performed in 24-well plates. However, researchers no longer need to take the culture plates out at estimated timepoints for sampling, or add any additives to dye the cells for measurements. Instead, when cultivating in S.NEST microbioreactors, the built-in real-time DO, pH, OCR, and ECAR monitoring provides real-time feedback to the researchers. The cultivation environment remains secure and sterile throughout the entire experiment, eliminating the risks of contamination from sampling disturbances.
These advantages are illustrated with another example experiment: A549 cells were seeded at the same density of 5 × 104 cells/well but in a 24-well S.NEST sensor plate, and treated with the same therapeutic drugs, MTX and SSP. The cytotoxicity of the drug were monitored through real-time DO, pH, OCR, and ECAR values.
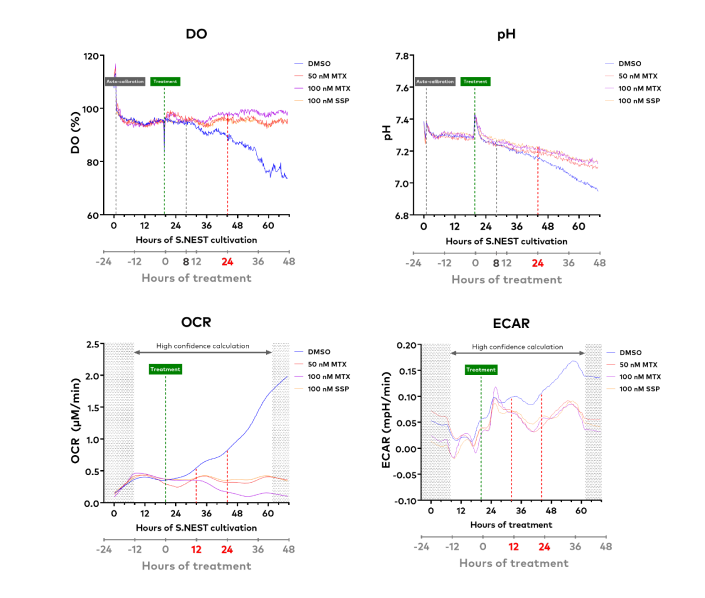
Significant differences in DO and pH curves between control and treated groups were observable within 24h of treatment. These differences indicate the time period where cells in control group were still proliferating while the drug treated cells experienced limited growth, induced cell death, and decreased cellular metabolic activity. Moreover, the differences between treatment and control groups can be detected even earlier with the OCR and ECAR values, which were discernable within 12h of treatment. With this experimental workflow, treatment reaction time can be determined in just one round of experiments by performing follow-up analysis of this time series data.
Conclusion
The S.NEST microbioreactor combines small scale mixing culture with real-time measurement of DO, pH, OCR, and ECAR values. S.NEST can evaluate a large number of samples treated with different drugs within a short timeframe, making it suitable for high-throughput drug screening.
Real-time tracking
of in vitro cell growth

Higher efficiency and
accuracy than end-
point cell viability
assay

Identification of the
time frame where
cytotoxic processes
occur

Enabling the
assessment of time-
dependent effects

Rapid and noninvasive
drug screening procedure
for assessing cytotoxicity
Dyefree procedure
eliminating the need for
additional cell-based assay

No need to predetermine
sampling timepoints using
sacrificial samples



