Introduction
Microbes are the one of the expression systems for recombinant proteins and plasmids, which is faster and less expensive method than mammalian expression system. The bacteria expression system, especially E.coli is the commonest as expression host for protein production since its cost-effective culture system as well as protein productivity. Yeasts are also the common choices due to its capability of post-translational modifications.
The dissolved oxygen is an important index for scaling-up microbes. C.BIRD is a high throughput microbioreactor, its mixing culture increases oxygen transfer efficiency for high oxygen of demand of cell lines.
Introduction
Microbes are the one of the expression systems for recombinant proteins and plasmids, which is faster and less expensive method than mammalian expression system. The bacteria expression system, especially E.coli is the commonest as expression host for protein production since its cost-effective culture system as well as protein productivity. Yeasts are also the common choices due to its capability of post-translational modifications.
The dissolved oxygen is an important index for scaling-up microbes. C.BIRD is a high throughput microbioreactor, its mixing culture increases oxygen transfer efficiency for high oxygen of demand of cell lines.
PERFORMANCE DATA
Improving Oxygen Transfer in Standard 96-well Plates Using the C.BIRD Microbioreactor

Diagram of experiment design
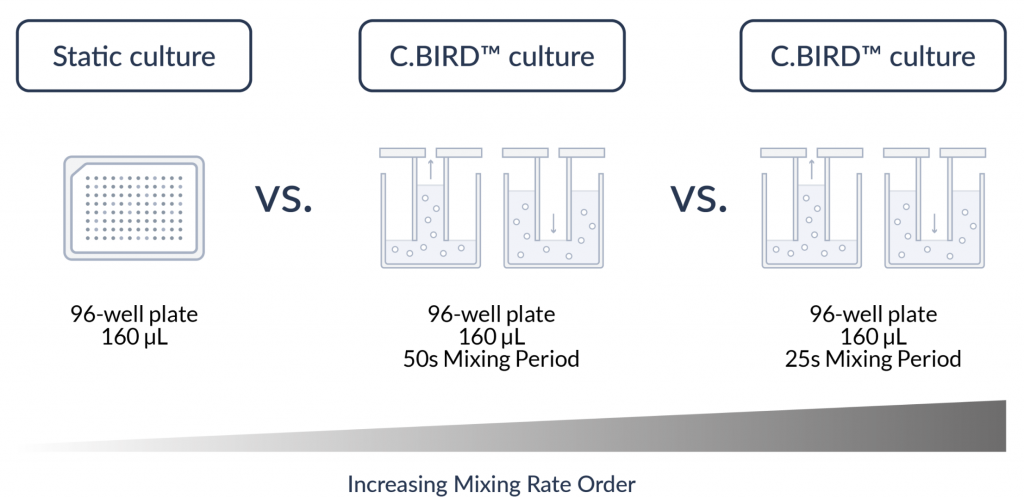
Olympus cellSens software was used to measure the fluorescence intensities. A 340 to 390 nm excitation filter and a 692/40 nm emission filter were used. Samples were exposed for 340 milliseconds and were read three times per interval.
C.BIRD’s mixing cycle was stopped 15 seconds before each reading to ensure the same fluid level for every measurement. Comparison studies were performed with samples measured in static conditions and in the C.BIRD at different mixing periods.
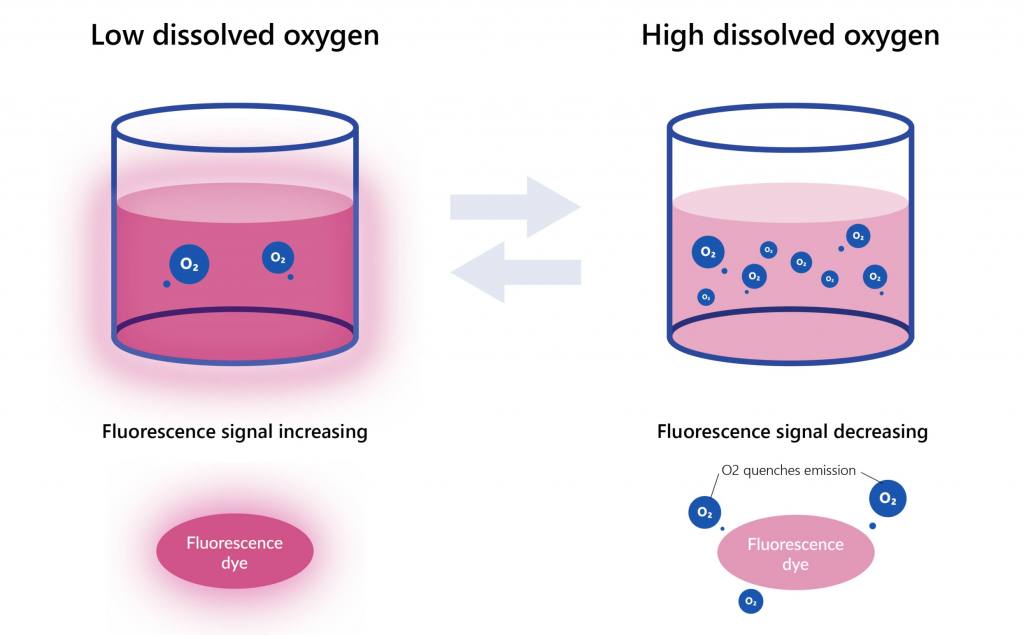
The principle of extracellular oxygen consumption assay kit
Conclusion
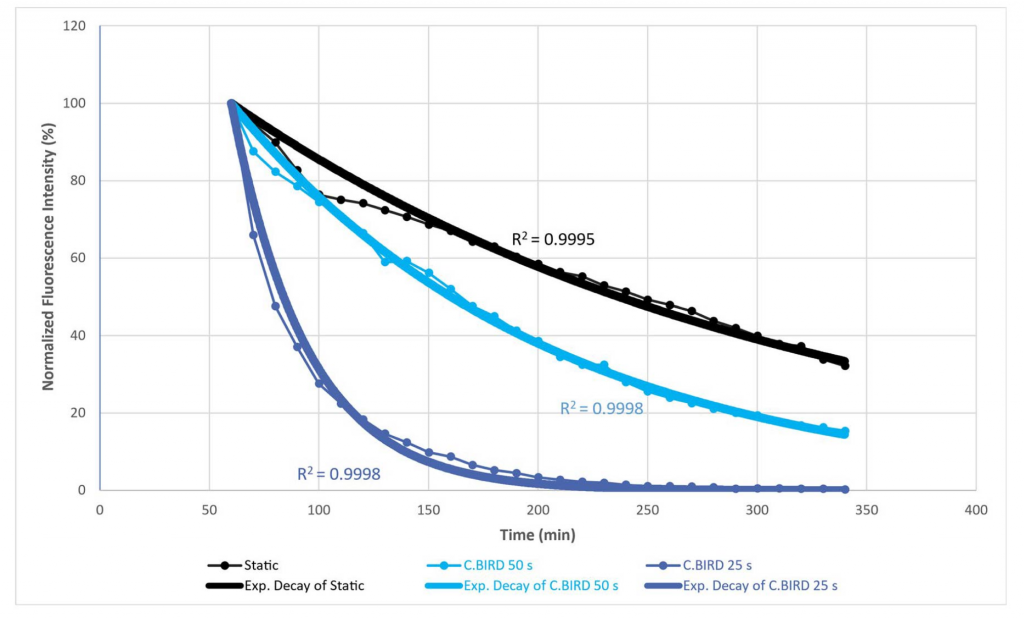
This study shows that the C.BIRD improves oxygen transfer in standard 96-well plates by comparing glucose depletion and oxygen recovery in different mixing conditions. The exponential decay observed in the experiment showed that the time constants were shorter for C.BIRD 50-second and C.BIRD 25-second conditions than for the static condition.
This translates into an oxygen transfer rate that is twice as fast in C.BIRD 50-second and up to 7 times faster in C.BIRD 25-second when compared to the static condition.

High-throughput Screening of Recombinant Clones in Microscale
Diagram of experiment design
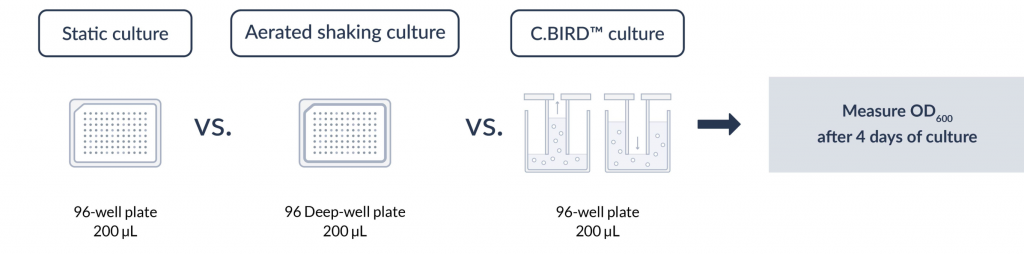
The measurement of OD600 in each well indicated the cell growth under static, aerated shaking culture and the C.BIRD culture conditions.
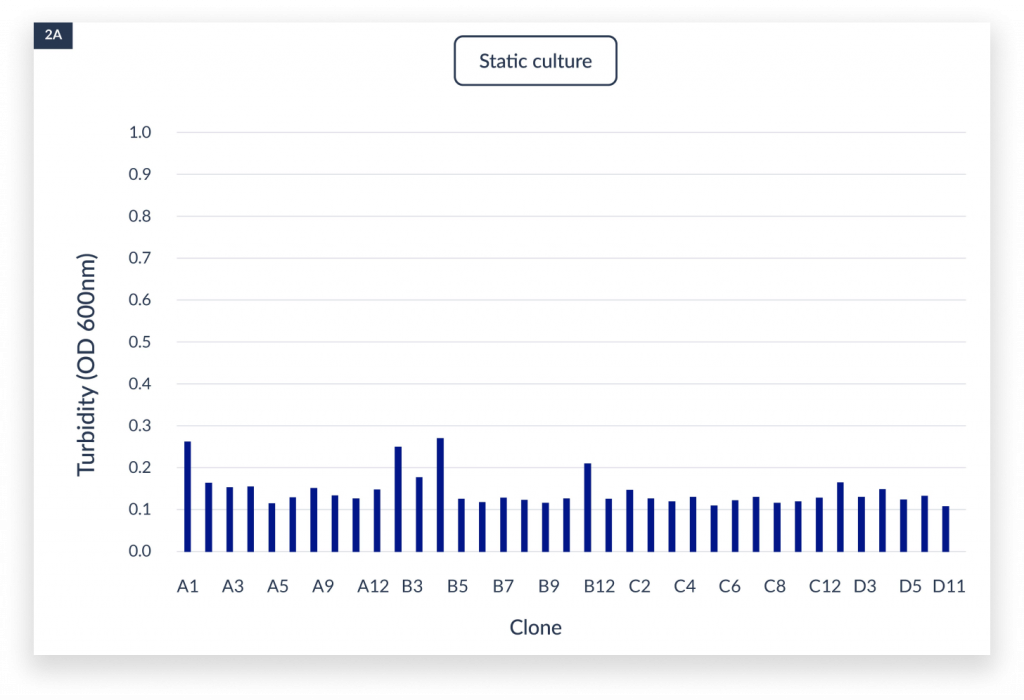
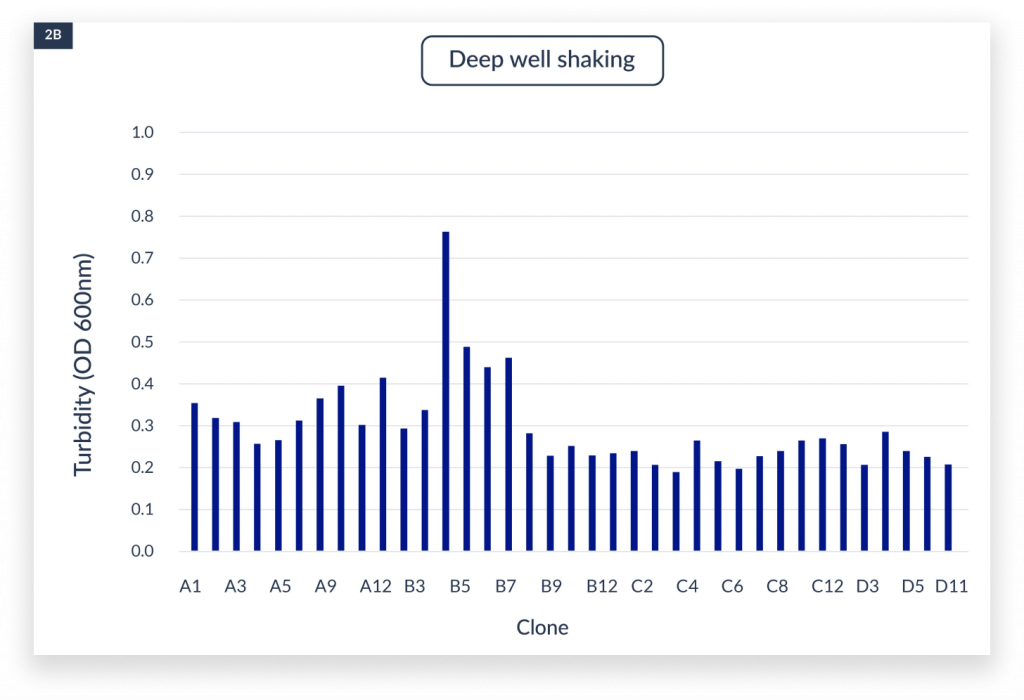
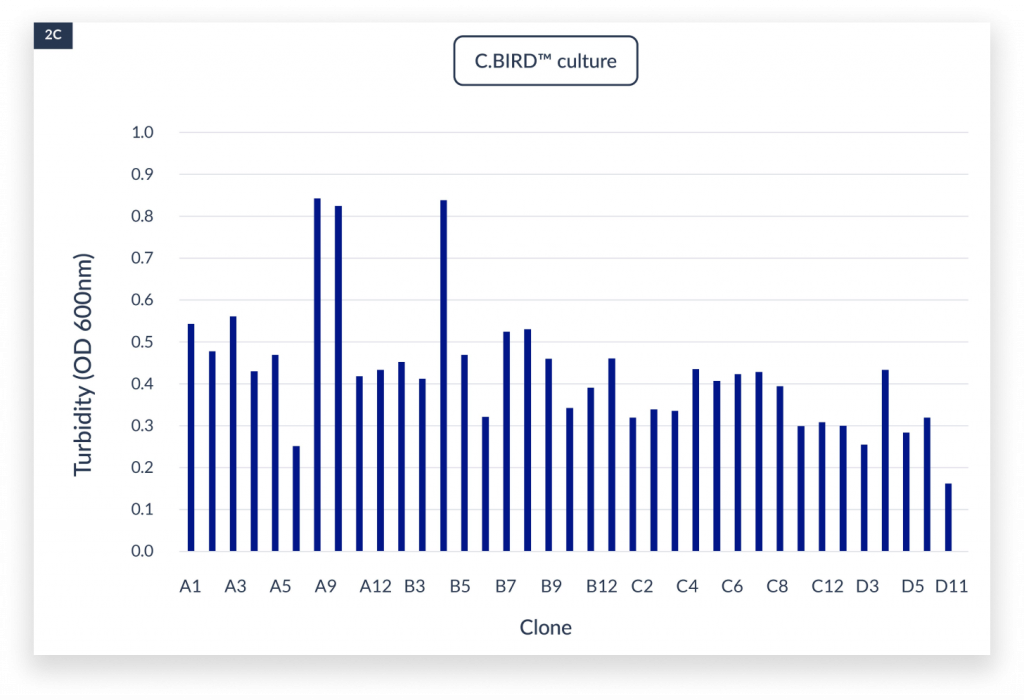
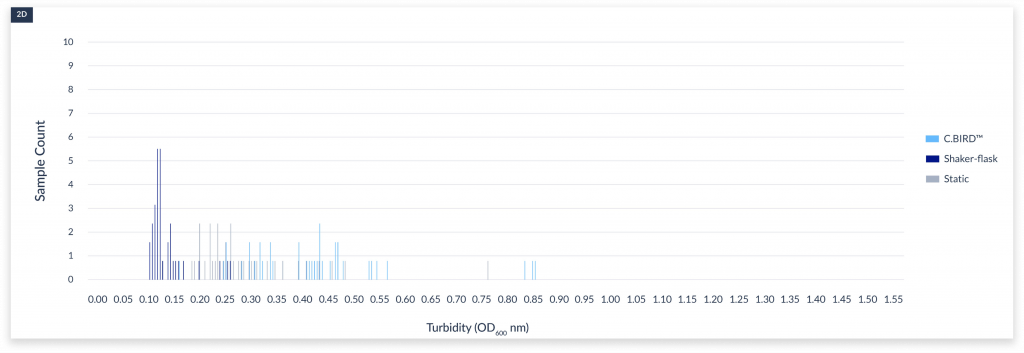
The cell densities under three culture methods after 4 days of culture. The x-axis represented the name of the clones. A)
static culture, B) aerated 96-DW shaking culture, and C) C.BIRD culture were shown. D) Integrated data showed the distribution of turbidity and its sample counts of the three culture methods.
Conclusion
Introducing the C.BIRD method to standard 96-well plates provided higher oxygen transfer rates, which
furnished the aerobic cell culture with better culture conditions. Our results show that efficient mixing during the P. pastoris cell culture process dramatically and evenly elevated cell density, allowing researchers to select highly productive clones in a high-throughput fashion, with no need for laborious process.



