
From Orbital Shakers to Automated Microplate Agitation:
Observations on an Evolving Technology
Observations on an Evolving Technology
Shifts in Cell Line Development Needs Following the Pandemic
The biopharmaceutical industry has seen significant shifts in cell line development (CLD) workflows following the COVID-19 pandemic, with a growing emphasis on high-throughput screening and faster early-stage process development [1]. Traditional methods like orbital shaking have struggled to meet new demands for efficiency and tighter control, prompting a move toward automated, miniaturized systems for scalable early cultivation [2]. Continuous biomanufacturing approaches are also being explored to streamline timelines and reduce variability, particularly for monoclonal antibody production [3] [4].
Reevaluating Agitation in Early-Stage Clone Screening
Agitation plays a key role in early clone screening by promoting uniform nutrient and oxygen distribution and effective waste removal, all critical to cell growth and viability [5]. Suboptimal agitation can lead to stress and variability, making it harder to accurately select the right clone selection. Despite its importance, early-stage agitation is often neglected. A survey showed that almost all companies only introduce agitation during the deep-well plate or flask stages, meaning cells often remain in static culture conditions for three weeks or even longer [6]. To address this gap, agitation should be implemented earlier and uniformly across culture scales to better reflect production-relevant conditions [3]. This shift can improve clone ranking accuracy and reduce downstream risks.
Technical Constraints of Orbital Shaking at the Microliter Scale
Orbital shaking faces inherent challenges at microliter volumes in microplate formats. Uniform mixing becomes difficult due to fluid dynamics and surface effects. Studies in 24 deep-well plates have shown significant evaporation and osmolality differences among wells, especially after prolonged incubation [5]. Inadequate oxygen transfer compounds the issue, leading to gradients and reduced viability at high cell densities.
Volumes with hundreds microliter are especially problematic, requiring high energy to overcome surface tension [2]. Orbital shakers, originally designed for larger flasks, often fail to provide sufficient agitation at this scale. As a result, early-stage cultures are frequently left static, undermining gas exchange and nutrient uniformity. These technical limitations underscore the need for new agitation technologies suited to small-volume, high-throughput formats [5].
Recent Advances in Microplate Agitation Culture Systems
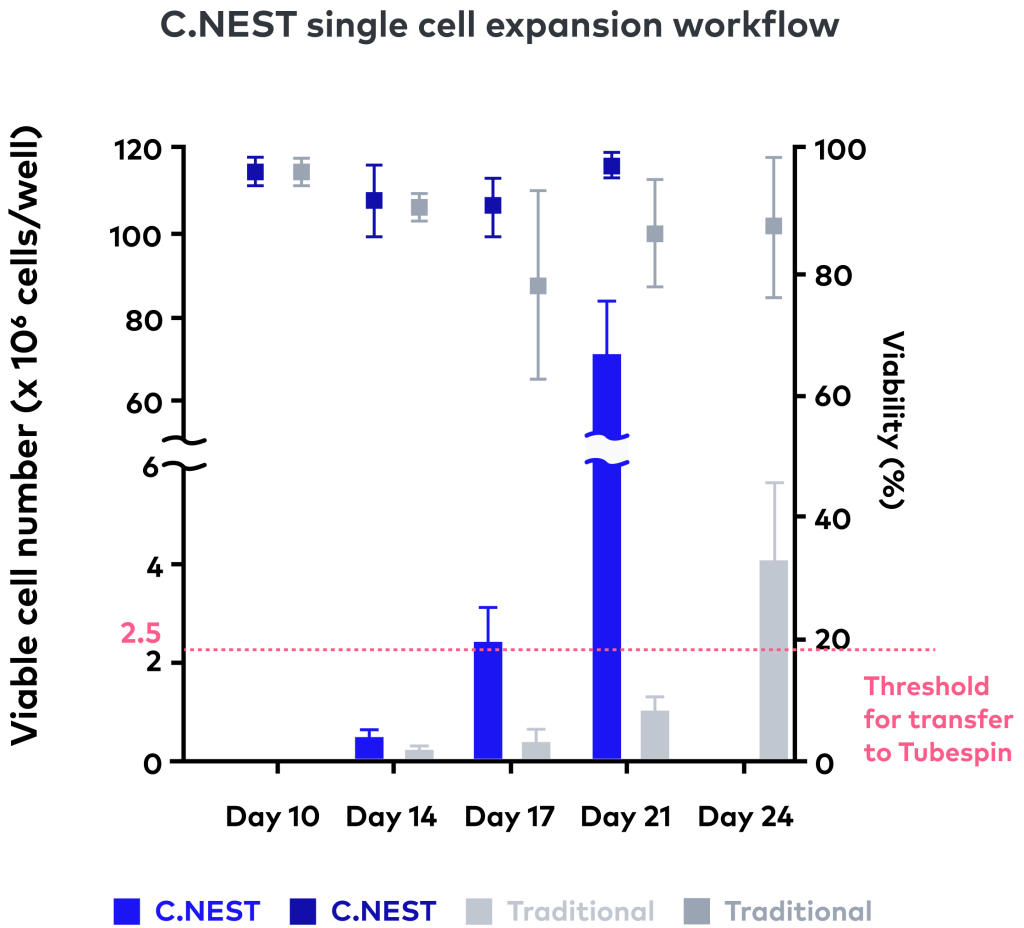
Conventional orbital shakers often fall short in microplate formats, especially at low volumes where uneven mixing and evaporation degrade culture consistency. CYTENA BPS developed the C.NEST system to address this gap, offering controlled, in-well agitation optimized for 96- and 24-well plates. C.NEST uses a reciprocating, bidirectional mixing mechanism with precise control over speed and frequency, enabling consistent agitation at volumes as low as 150–200 µL. A patented oxygen transfer lid enhances gas exchange directly within each well, supporting robust and reproducible cell growth.
Further ensuring culture consistency, the system integrates independent incubation chambers that regulate temperature, CO₂, and humidity, with UV sterilization and built-in water reservoirs to minimize evaporation. In CHO-S cell line development workflows, adopting C.NEST during 96- and 24-well plate stages resulted in approximately 30% faster progression to TubeSpin transfer—saving around seven days—without compromising cell doubling times [7]. Together, these features position C.NEST as a high-throughput, automation-ready solution that supports reliable clone selection and accelerates early-stage CLD processes.

Summary and Perspectives
As biopharmaceutical timelines compress and process complexity increases, the shortcomings of static and orbital mixing—such as inconsistent gas exchange and nutrient distribution—become more detrimental to early-stage decision making. These inconsistencies impact clone selection reliability and downstream process predictability.
C.NEST responds to this challenge with programmable, in-well agitation and stable microenvironment control tailored for small-volume, high-throughput formats. Its demonstrated ability to compress development timelines while maintaining cell health aligns with a broader shift toward modular, automation-ready upstream systems. As selection strategies grow more multidimensional, early predictive screening enabled by such systems will be pivotal. Controlled agitation at the microplate level is no longer a luxury but a foundational requirement for next-generation CLD workflows.
References
[1]J. Hemmerich, S. Noack, W. Wiechert, M. Oldiges, and M. Oldiges, “Microbioreactor Systems for Accelerated Bioprocess Development,” Biotechnology Journal, vol. 13, no. 4, p. 1700141, Apr. 2018, doi: 10.1002/BIOT.201700141.
[2]L. J. Frey and R. Krull, “Microbioreactors for Process Development and Cell-Based Screening Studies,” Advances in Biochemical Engineering/Biotechnology, vol. 179, 2020, doi: 10.1007/10_2020_130.
[3]M. Dorn, C. Ferng, K. Klottrup‐Rees, K. Lee, and M. Micheletti, “Cell clone selection—impact of operation modes and medium exchange strategies on clone ranking,” Frontiers in Bioengineering and Biotechnology, vol. 12, Jan. 2025, doi: 10.3389/fbioe.2024.1479633.
[4]M. Tregidgo, C. Lucas, M. Dorn, and M. Micheletti, “Development of mL-scale pseudo-perfusion methodologies for high-throughput early phase development studies”.
[5]S. Ohira and T. Omasa, “Incorporating shaken 24-deep-well plate fed-batch culture shortens CHO cell line development time,” Cytotechnology, vol. 77, no. 2, p. 64, Apr. 2025, doi: 10.1007/s10616-025-00728-4.
[6]H. Clarke et al., “When will we have a clone? An industry perspective on the typical CLD timeline,” Biotechnol Prog, vol. 40, no. 4, p. e3449, Jul. 2024, doi: 10.1002/btpr.3449.

